Abstract
Background Azacitadine (AZA) is a cytidine analogue that incorporates into replicating DNA or RNA and binds to DNA methyltransferase (DNMT1) leading to its degradation and global DNA hypomethylation. The optimal dosing of AZA for DNA hypomethylation without overt cytotoxicity is not well understood. We used a novel flow cytometry-based assay to measure DNMT1 in leukemia cells as a pharmacodynamic marker of intracellular drug targeting in patients receiving low dose AZA for relapsed AML/MDS post hematopoietic cell transplantation (HCT).Disease relapse is the most common cause of treatment failure post-HCT with no established standard of care. Time to relapse is the major factor impacting outcome and survival in those with relapsed disease within 6 months is estimated to be less <5% (Bejanyan N etal BBMT 2015;21(3):454-9).
Methods We retrospectively reviewed 29 AML and MDS patients (median age 63.5 years, range 28-74) who received low dose intravenous AZA for treatment of disease relapse at a median of 105 days (range 23-1477) after allogeneic HCT from 2009 to 2016. All were considered ineligible for second transplant due to early relapse or poor functional status. Median AZA dose was 40 mg/m2/day x5 days (range 16-100), determined per clinician preference. We assessed DNMT1 in CD34+/CD117+ blasts in blood or marrow in 8 patients with available samples. We previously reported a novel S-phase specific flow cytometric DNMT1 assay to measure the direct pharmacodynamic effects of AZA (William B etal BBMT, Volume 21, Issue 2, S318 - S319). Cryopreserved mononuclear cells from patient blood or marrow were thawed and fixed using formaldehyde (1%) and methanol (90%). Fixed cells were stained for DNMT1 and cell surface antigens. Cell cycle analysis was performed using DAPI staining. The expression of DNMT1 in leukemia cells in S-phase was measured as mean fluorescent intensity (MFI) normalized to a control.
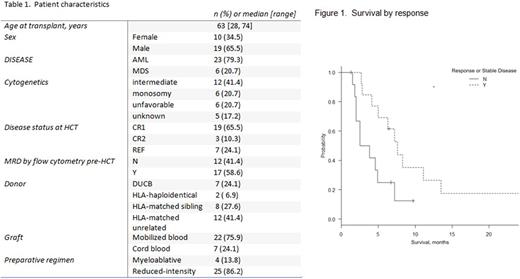
Results Patient characteristics are summarized in Table 1. Disease relapse occurred at a median of 105 days (range 23-1477) after HCT. Median starting dose of AZA was 40mg/m2 for 5 or7 days of every 28 day cycle. Median number of AZA cycles administered was 3 (range 1-19). Ten patients received at least one DLI (median DLI per patient=2, range 1-5). With a median follow up of 6.3 months (range 1.3-41.1 months), 7 patients (27%) responded to AZA with complete remission (CR), hematologic improvement, or improvement in donor chimerism. Additional six (21%) patients exhibited stable disease. One patient achieved a sustained CR >2.5 years despite continued immunosuppression due to ongoing chronic graft versus host disease.
Median survival was 6.8 months (95% CI 3.8-11.1). Any response to AZA was associated with a higher survival (7.6 vs 3.2 months, p=0.026) (Figure 1). Survival was not significantly different between the two AZA dose groups (<40 or >40mg/m2/day, OS 7.6 vs 4.9 months, p=0.5).
Eight patients (3 MDS, 5 AML) had available samples (marrow or blood) at baseline and after initiation of AZA. Four patients with AML had a subsequent decrease in DNMT1.Two patients with MDS had in increase in DNMT1 after initiation of AZA, however the samples were early in the treatment course (C1D3 and C1D15). Given the heterogeneity in sampled time points in relation to therapy, correlation to clinical response is unable to be determined. Within these limitations, a higher pre-treatment DNMT1 level and notable decrease was observed in the AML patient (Subject 2) who achieved CR from hematological relapse sustained for 3 years. In the remaining 7 patients who did not respond or had a short duration of response, the variation in DNMT1 level pre and post-treatment was minimal.
Conclusion Within the limitations of a small retrospective study, our data suggest that low dose AZA is comparable to high dose AZA for treatment of AML /MDS patients who relapse post-HCT. A significant proportion of this poor- risk population, with median time to relapse of 105 days, responded to low-dose AZA. Furthermore, we obtained serial quantification of DNMT1 levels throughout AZA therapy using a novel flow cytometry assay. One patient with sustained response exhibited high baseline DNMT1 level and decrease after therapy. These results suggest that lower doses of AZA may achieve similar therapeutic efficacy with less myelosuppression; however, this hypothesis should be further evaluated in the standard dose setting.
Caimi: Seattle Genetics: Equity Ownership; Incyte: Equity Ownership; Abbvie: Equity Ownership; Celgene: Speakers Bureau. Cooper: Novartis: Research Funding. Little: Hemex Health: Equity Ownership. Malek: Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees. De Lima: Celgene Corporation: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal